Ethics in the CGI-Clinics project
This page describes how patients are involved in the CGI-Clinics project, and how we ensure that we use patients’ perspectives and data in an ethical way.
How do patients contribute to CGI-Clinics?
Patients are a crucial part of the CGI-Clinics project as our research advancements are not possible without their participation. The goal of the project is to improve cancer genome interpretation, which involves understanding the impact of mutations in cancer cells, to be used for the benefit of patients and society.
The project includes several studies, involving different research teams, in which patients participate in different ways. Patients may participate directly by critically reviewing our tools and resources or indirectly by sharing their data for cancer research.
Stay updated on CGI-Clinics
How can patients stay abreast of the progress of the CGI-Clinics project and research done with their data?
The conclusions and discoveries of the CGI-Clinics collaborating partners will be disseminated to the scientific community through publications and conferences and will also be communicated to the general public and patients through news on the project’s website, email newsletter, and social media.
Click or tap the tabs below to find out about the different ethical aspects of the project.
Is the CGI-Clinics project safe for patients?
The CGI-Clinics project makes every effort to ensure the safety and integrity of the cancer patients’ data during their participation in the studies. Similarly, we treat sensitive data according to data privacy laws (i.e. GDPR), minimising the possibility to re-identify people based on their genomic information and identifiers. To achieve this, we follow specific procedures and protocols that ensure an ethical application of the studies. Additionally we count on an ethics advisor who oversees compliance.
Participants always have the right to change their minds and withdraw consent at any stage of the studies. Additionally, when participants agree to join in a study, they are not expected to receive any direct health benefits; instead, their participation contributes to improve the social impact of research.
What is an ethics protocol?
An ethics protocol is a set of guidelines designed to guarantee that research or professional practices are conducted ethically and responsibly. It encompasses principles such as informed consent, confidentiality, risk assessment, integrity, and accountability, ensuring that participants understand their involvement, protecting their privacy, minimizing risks, and maintaining honesty in reporting. An ethics protocol includes several components:
– Informed Consent: Ensuring participants understand the study and voluntarily agree to participate.
– Confidentiality: Protecting the privacy of participants and the data collected.
– Risk Assessment: Identifying potential risks to participants and outlining measures to minimise them.
– Integrity: Maintaining honesty and transparency in reporting results and methodologies.
– Accountability: Clarifying the responsibilities of researchers and practitioners regarding ethical standards.
How is the ethics protocol approved, and who approves it?
Ethics protocols are approved by Ethics Review Committees (ERCs). The ERCs comprise a multidisciplinary group of professionals. The approval process involves researchers submitting their protocol, detailing the objectives, methods, and ethical considerations of the study. The ERC reviews the protocol, provides feedback as needed, and then either approves, conditionally approves, or rejects it. Once the protocol has been approved, ongoing monitoring may be required to ensure continued compliance with ethical standards throughout the research process.
In the case of IRB Barcelona (project coordinator and promoter of the studies presented) this role is fulfilled by the Ethics Committee for Drug Research (CEIm of the Hospital Clínic de Barcelona). Also, the hospital sites of all CGI-Clinics partners involved in a study have to review and approve the protocols.
What is an ethics advisor?
An ethics advisor is a professional who provides guidance on ethical issues, ensuring that studies comply with ethical standards and guidelines. They provide expert consultation on ethical dilemmas that researchers may face, such as issues related to informed consent, participant privacy, and potential risks. Ethical advisors prioritize the rights and welfare of participants throughout the research process.The contributions of these advisors are essential in fostering a culture of integrity and accountability in research, ultimately enhancing the credibility and reliability of the findings.
What research studies is CGI-Clinics conducting?
The following sections explain the CGI-Clinics studies involving patients and provide information on where to find updates and results from the research performed. Each study has different objectives and a different involvement.
STUDY 1: CGI-Clinics: Assessment of the patient’s experience with genomic and mutational testing of cancer to empower patients in the decision-making process
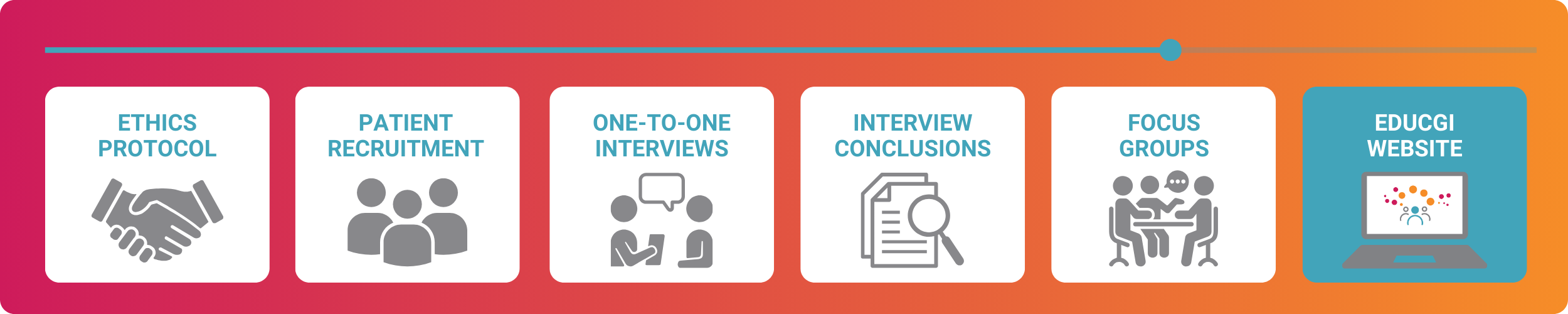
New tests assessing cancer genome profiling and biomarkers are progressively used in diagnostic processes in hospitals. Certain technical aspects can be challenging for patients to understand. The CGI-Clinics initiative seeks to empower patients by enhancing their understanding of these concepts, enabling them to communicate more confidently with their doctors and becoming active participants in scientific research.
Study 1 aims to identify patients’ needs and concerns related to cancer genome interpretation and critically evaluate the main tools and resources for patients generated within the CGI-Clinics project. It involves a small group of patients through interviews and group discussions.
Why do patients’ perspectives matter?
We believe that patients have valuable insights. In our case, by sharing their experiences and perspectives, they can help researchers improve data managing for research purposes and contribute to the development of new tools. Ultimately, this ensures that the efforts of CGI-Clinics will directly benefit cancer patients in the future and make sure that the outputs of the project fulfill their needs and concerns.
How have patients been involved?
In 2024, we have conducted interviews with patients to gather information about their experiences and concerns regarding genomic data-sharing and their understanding of biomarker testing. These interviews aim to describe the patient journey, highlighting experiences from the diagnosis of cancer to treatment, awareness of data sharing, impact of testing on quality of life, and identification of gaps in knowledge or support.
At the end of 2024, we will perform two separate Patient Advisory Boards, in which patients will provide feedback on our tools and resources to ensure they are tailored to meet everyone’s needs.
What is the next step?
The conclusions of Patient Advisory Boards will be drawn up into guidelines and will directly influence the development of the following resources within CGI-Clinics:
- CGI-Clinics website: making sure that the website is accessible
- CGI-Clinics: developing guidelines which will ensure that the clinical CGI tool is patient-centric.
- EduCGI: creating guidelines which will ensure that the EduCGI website meets patients’ needs.
STUDY 2: CGI-Clinics: Data-driven decision support tools for better healthcare delivery and cancer-focused policy making.

What is the study about?
CGI-Clinics aims to improve the implementation of molecular testing in oncology through the optimization and validation of a data-driven decision support tool (the Cancer Genome Interpreter; CGI). CGI-Clinics ambitions to increase access to homogeneous and more complete genomic data interpretation (after sequencing and before advising on compatible targeted therapies) for each cancer patient across hospitals, regardless of their resources, while empowering the patient to become an active actor in the process and providing policy-makers aggregated statistics on actionable cancer genomic variants.
Interpretation is a bottleneck for the full deployment and broad accessibility of Next Generation Sequencing (NGS) in cancer management.
Study 2 seeks to improve the interpretation of the mutations found in the DNA of the tumour using the CGI tool. While this tool was originally designed in a research context, we are developing a clinically oriented version to support medical decisions made during diagnosis and treatment.
In practical terms, the study involves an additional analysis of patients’ genomic data for research and educational purposes, without any modification of treatments.
The study has two main objectives: to help clinicians to learn more about biomarker testing and interpretation; and to help researchers improve methods for the interpretation of the genomic data.
Why is patient participation so crucial?
The CGI tool is based on data-driven methods and machine-learning algorithms. These types of algorithms need real-life data to improve their accuracy. The CGI tool learns from thousands of patients, and therefore has been built through the altruistic sharing of data by those patients. Thanks to the generosity of current and future study participants, the prediction power and interpretation precision of the CGI tool will be enhanced.
The methods are being continuously improved as more high-quality open data sets become available and accessible through appropriate research agreements.
Each patient who shares their data is essential for improving genomic data interpretation and contributes to the progress of precision medicine.
What data does CGI-Clinics use?
The project uses genomic information of cancer cells provided by Next Generation Sequencing (NGS) analysis. This analysis looks at the genomic composition of tumour samples or blood tests to identify specific alterations.
Although this data is typically gathered during routine medical tests, patients are asked to consent to the re-use of their data for research purposes to improve the algorithms of the CGI tool. These improvements will impact the interpretation of genomic information of the tumours of future patients -as we are generating regular updates of the algorithms.
In this context, informed consent ensures that patients are fully aware of how their data will be used while respecting their right to privacy and autonomy in medical research.
What does CGI-Clinics do with data provided by patients?
During the genomic data analysis, the data is kept on a safe server with restricted access. The identity of each patient is preserved by means of codification (only their hospital has the code).
There is a primary and a secondary purpose in using patients’ genomic and clinical data.
● Primary: The healthcare professionals analyse the tumour profile and some clinical data with the CGI tool to interpret the molecular profile of the tumour. Access to this personal information is restricted to the clinician involved in the study and the research team.
● Secondary: Part of the clinical and genomic data (without the patient codes) is shared with the Biomedical Genomics Lab at IRB Barcelona, where a small number of researchers have restricted access to it to improve the computational methods and algorithms for cancer genome interpretation. In this case, medical information cannot be traced back to the patient, which means that their data is anonymized, or pseudonymized when the characteristics of the data does not allow complete anonymization.
NEXT STEPS: Patient perspective
CGI-Clinics recognises the importance of incorporating patients’ perspectives into the different phases of the project. In this regard, the next step will be to incorporate patients’ perspectives into the virtual Molecular Tumour Boards (vMTBs).
What is a Molecular Tumour Board (MTB)?
A Molecular Tumour Board (MTB) is a group of different types of experts that analyses complex tumour cases to help oncologists recommend the right therapeutic options for a patient based on the molecular and clinical data. The CGI-Clinics project is implementing a virtual Molecular Tumour Board (vMTB) where international experts from areas like molecular pathology and clinical oncology will meet online to interpret critical data for challenging cases such as complex molecular profiles.
The MTB experts are expected to commit to the virtual tumor board by reviewing the proposed cases and attending the scheduled meetings.
The activities that will be carried out in the MTB are the following:
1. Provide expert opinion on interesting genomic findings and their clinical relevance.
2. Considering the genomic report, recommend whether a patient could be offered a matched targeted therapeutic recommendation.
3. Provide information regarding clinical trials
4. Advise in case of issues with the tests performed.
How can patients participate in these MTBs?
We will soon invite representatives from patient associations and patient advocates to attend our vMTB meetings online. Their presence will provide valuable insights and perspectives on reviewed cases. This initiative aims to enhance communication, empower a patient-centric perspective, and improve patient access to information, ultimately enriching the decision-making process. All data presented in the MTB will be codified and all members will sign a specific agreement regarding data management and ethical regulations.
This is what we would like patients to know:
The CGI-Clinics team is most grateful to patients for sharing their data. This has greatly helped us to make significant progress in understanding cancer at the molecular level, through advanced computer-based methods like those used by CGI. The CGI-Clinics collaborating partners are working to bring these methods into clinical settings to better understand and treat each type of cancer.
We are dedicated to protecting patient rights. That is why we limit the collection of sensitive data for the main use of the CGI tool and further anonymize it for research purposes. Anonymizing data provided by patients means that information about their health cannot be traced back to them, as sensitive personal details and any codes that could identify the individual are removed.
We support the safe use of patient data in research to improve the quality of cancer research, ultimately benefiting patients and society.
Stay updated on CGI-Clinics
How can patients stay abreast of the progress of the CGI-Clinics project and research done with their data?
The conclusions and discoveries of the CGI-Clinics collaborating partners will be disseminated to the scientific community through publications and conferences and will also be communicated to the general public and patients through news on the project’s website, email newsletter, and social media.

